
Introduction:
Have you ever wondered about the mind-boggling world of chiral compounds? Among them, helicenes are attracting attention due to their potential in cutting-edge chiral photonics applications, such as circularly polarized OLEDs and CP-selective transistors. Their unique helical structure allows for the possibility to tune their optical and chiroptical properties through chemical synthesis.
A recent study delved into the relationship between ground and excited state properties of helicenes, analyzing absorption (gabs) and luminescence (glum) dissymmetry factors for 170 helicenes and related compounds. The goal? To verify the predicted glum/gabs relationship, which could streamline the estimation process and help identify key differences in ground/excited state geometry.
So, if you're intrigued by the fascinating world of helicenes, read on for more insights! 🚀
What is g(lum) and g(abs)?
In the world of chiral molecules and their interaction with light, two essential parameters play a vital role in understanding their behavior: g(luminescence), or g(lum), and g(absorbance), or g(abs).
g(luminescence), abbreviated as g(lum), refers to the dissymmetry factor associated with the luminescence of chiral molecules. Luminescence is the emission of light by a substance that has absorbed energy, such as from a photon. The g(lum) factor quantifies the difference in intensity between the left and right circularly polarized components of the emitted light. In other words, it indicates how much the luminescence process is affected by the chiral structure of the molecule.
On the other hand, g(absorbance), or g(abs), represents the dissymmetry factor of the absorbance process. Absorbance is a measure of the amount of light absorbed by a substance as it passes through a sample. In the context of chiral molecules, g(abs) quantifies the difference in absorbance of left and right circularly polarized light by the chiral molecule. It helps researchers understand the extent to which the chiral structure of the molecule influences the absorption of light.
Both g(lum) and g(abs) are crucial parameters for characterizing chiral molecules and their interactions with light. By comparing and correlating these values, researchers can gain insights into the optical properties of chiral molecules and explore potential applications in areas such as fluorescence imaging, chiral recognition, and asymmetric catalysis.
Key findings:
In the world of helicenes, these fascinating molecules are formed by fused rings and come in various sizes, from [4]-helicenes to [11]-helicenes. Their unique structures and properties make them useful in applications such as cell fluorescent stains and spin-filtering chiral systems. The number preceding helicene (ex. 4, 11) has to do with the number of benzene rings fused together to form the rigid core of the molecule.
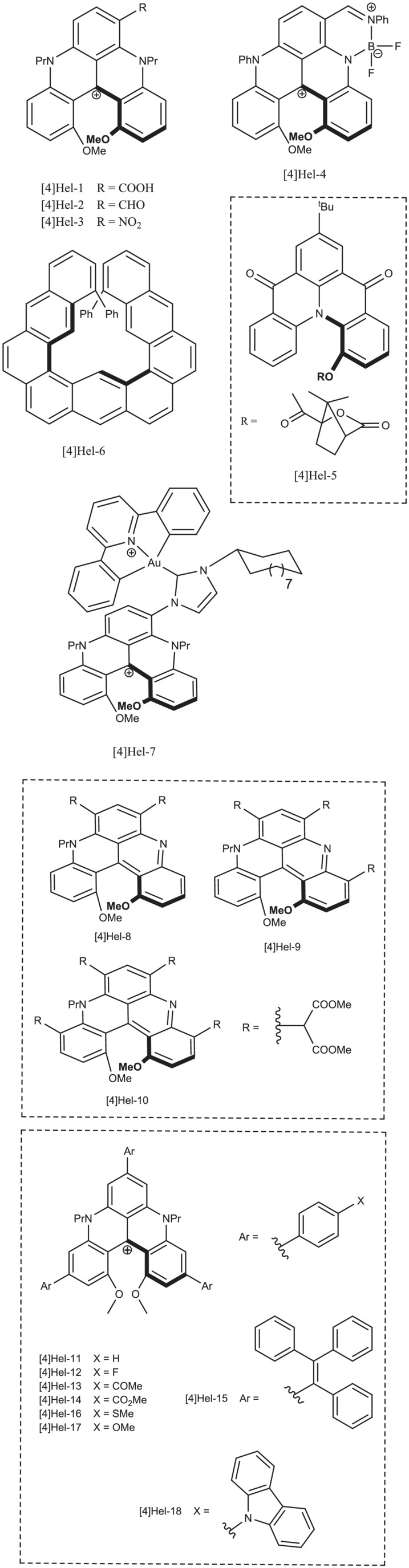

When diving into the realm of [4]-helicenes, you'll find a strong correlation between their g(lum) and g(abs) values, which helps us understand their fascinating properties. As we venture further into the larger [5]-helicenes, the diversity increases, and an interesting deviation is observed for [5]Hel-49 and 50. It is speculated that the different geometries of the emissive state are responsible for this deviation. [6]-helicenes, on the other hand, are stable at room temperature and exhibit a wide range of structures, with some notable deviations from the typical g(lum) and g(abs) ratios. Those deviations are typically found in the relatively simple and unsubstituted variants.
[7]-helicenes take structural complexity to new heights, with some even showing a whopping 500-fold enhancement of g(lum) factor when used as a chirality-inducing dopant. However, these molecules display a relatively poor g(lum) and g(abs) correlation, with four outliers causing the low correlation coefficient. Again, these are generally simple variants. Finally, the less studied [8]-[11]-helicenes still show a significant correlation between glum and gabs values, hinting at the potential for future exploration of their fascinating properties.
In a nutshell, helicenes come in various shapes and sizes, each with their unique characteristics and applications. They continue to captivate researchers, as the quest to understand their properties and potential applications unfolds.
Conclusion:
In conclusion, the comprehensive analysis of 170 helicenes, helicenoids, and heterohelicenes revealed valuable insights into the correlation between their ground and excited state chiroptical properties. The study showed that the g(lum) and g(abs) factors share a notable correlation for most of the examined compounds, despite their structural diversity. This correlation has significant implications, such as the ability to estimate g(lum) from ECD data, saving time and resources in computational calculations. Additionally, instances where g(lum) and g(abs)factors significantly deviate from each other could signal differences in ground/excited state geometry or the presence of alternative photophysical phenomena. The results of this research not only deepen our understanding of helicenes but also pave the way for further advancements in their applications, such as CP-OLEDs and CP-selective transistors.