
Unfortunately, the cement industry, a cornerstone of our global infrastructure, plays a significant role in our environmental challenges. Known for contributing to approximately 7% of worldwide carbon dioxide (CO2) emissions, the industry has long been looking for sustainable solutions.
Enter the groundbreaking study highlighted today: a game-changing process that combines electrochemical water splitting and CO2 reduction to transform limestone, a key ingredient in cement production, into calcium hydroxide (Ca(OH)2) and valuable carbon-based products. This innovative method could reduce CO2 emissions by an impressive 75% per tonne of cement produced.
A Fresh Take on a Chronic Issue
The study focuses on a pressing global concern: the rampant CO2 emissions contributing to global warming. With the cement industry being a notable emitter, any advancement in this sector could mark a significant step forward in our fight against climate change.
Historically, CO2 is released during the cement production process through the decomposition of limestone (CaCO3) to calcium oxide (CaO), the combustion of fossil fuels, and power consumption for related equipment. Recognizing these emission sources, several recent studies have explored methods to decarbonize cement production.
In an exciting development, this study presents an integrated approach for cement production that electrochemically converts limestone into calcium hydroxide and valuable carbon-based products. The process hinges on the in situ conversion of CO2 released during limestone decomposition, meaning CO2 is converted into beneficial products within the same system where it's generated.
The Dual-Chamber Triumph
At the core of this method lies a dual-chamber setup, whereby a positive current is applied to an electrode, triggering two separate reactions: the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER). These reactions are critical for continually producing calcium hydroxide and carbon-based products.
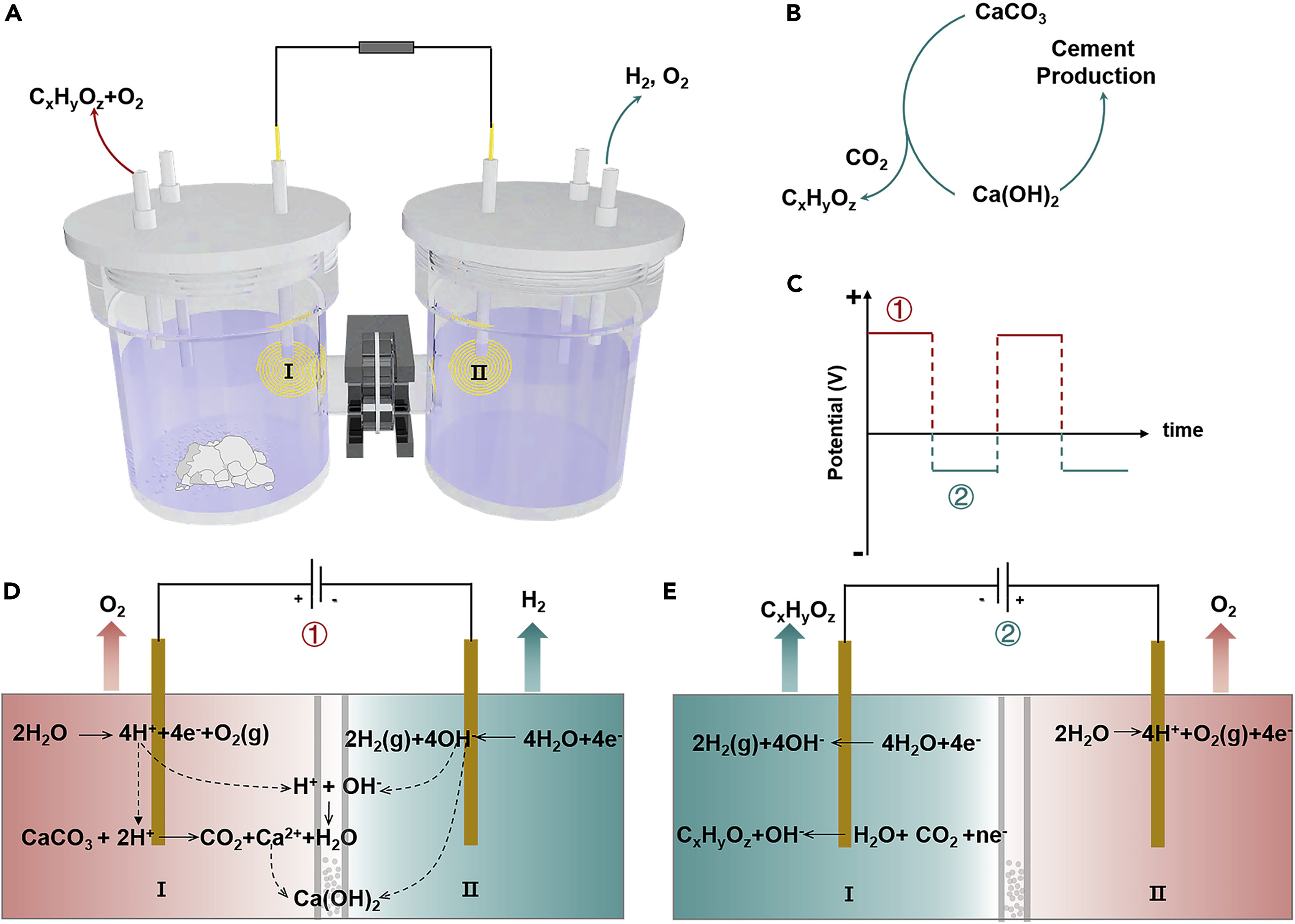

But the innovation doesn't stop there. Researchers took it a step further by developing a triple-chamber H-cell system, allowing the OER and CO2 reduction processes to occur on separate electrodes, thereby expanding the range of usable CO2 reduction electrodes.
The team conducted experiments using various metal electrocatalysts, such as silver (Ag), indium (In), and copper (Cu), all of which demonstrated successful transformation of limestone.
Towards a Sustainable Cement Industry
This research paints a hopeful picture for the future of cement production. Introducing a method that transforms CO2 emissions into beneficial carbon-based products, significantly curbs CO2 emissions from one of the world's most prevalent industries.
The study lays the groundwork for making substantial strides towards a net-zero or even negative carbon emission cement industry. This goal once seemed distant but is now inching closer to reality.
While the road to a fully sustainable cement industry is long and winding, it's studies like this that light the way, proving that with scientific curiosity and persistence, we can transform challenges into opportunities for innovation.
Stay tuned for more updates on groundbreaking research reshaping our industries and helping us build a sustainable future. Let's continue the conversation on creating a greener world – one tonne of cement at a time.
To complete your own summaries, register now for free access